While synthesizing your peptide sequence, it is crucial to design the experiment carefully to minimize any chances of failure. Fmoc-Glu(OtBu)-Thr[psi(Me,Me)Pro]-OH is a Fmoc amino acid derivative that ensures a perfect peptide sequence produced with high purity.
In this blog, we delve into its structural and functional details and its applications. Keep reading to learn more.
What is Fmoc-Glu(OtBu)-Thr[psi(Me,Me)Pro]-OH?
Fmoc-Glu(OtBu)-Thr[psi(Me,Me)Pro]-OH is a dipeptide having a molecular weight of 566.64 g/mol. Its chemical formula is C31H38N2O8,
and the chemical structure is:
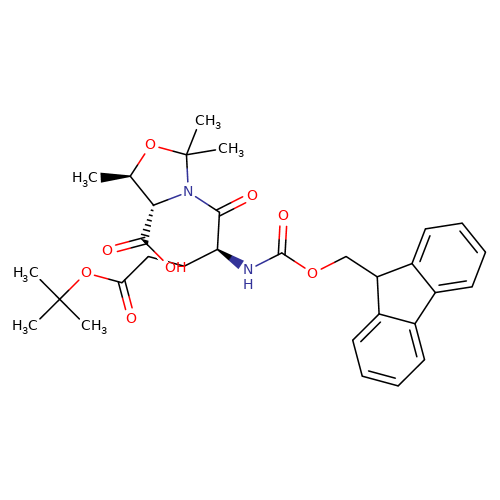
This compound has a fluorenyl methoxycarbonyl (Fmoc) protecting group, which is highly effective in synthesising solid-phase peptides. Its tert-butyl (OtBu) group enhances its stability and solubility, making it a go-to choice for researchers studying the synthesis of modified peptides with improved pharmacological properties.
It holds significant value in developing peptide-based therapeutics, especially where precise modifications can improve bioactivity and selectivity. Owing to its unique structure, it is used for designing drug delivery systems and targeted therapies. This dipeptide appears as a white or off-white coloured powder and needs to be stored under -4℃ to maintain its stability.
The Role of Fmoc-Glu(OtBu)-Thr[psi(Me,Me)Pro]-OH in Peptide Synthesis
This compound is designed to combat challenges often incurred during fmoc peptide synthesis, such as aggregation and solubility problems. Its pseudoproline moiety Thr[psi(Me,Me)Pro] provides structural rigidity and disrupts hydrogen bond networks, enabling efficient coupling of amino acids, even in sequences that are otherwise traditionally difficult to assemble.
The protective group, Fmoc, protects the α-amino group, allowing stepwise elongation of the peptide chain. The OtBu group shields the side chain carboxyl group of glutamic acid, ensuring orthogonal deprotection capabilities. This orthogonality allows us to gain precise control over the step-by-step assembly process, enhancing the overall reliability of the peptide synthesis reaction.
Also, the unique structure of this compound inhibits unwanted side reactions during the synthesis, like aspartimide formation, therefore holding prime importance when synthesizing biologically active peptides used in drug discovery and other studies.
Fmoc Peptide Synthesis: An Overview of the Process
Fmoc-based solid-phase peptide synthesis (SPPS) is the most commonly used method to synthesize peptides. In this method, Fmoc-protect amino acids are used, and the process is as follows:
Resin Preparation: Typically, resigns like Wang or Rink are used to anchor the first amino acid. The first amino acid is attached to the resin using DIC or HBTU activation.
Deprotection: As the name suggests, in this step, the amine group of the fmoc amino acid is exposed for subsequent coupling by removing the Fmoc group with a base like 20% piperidine in DMF.
Coupling: Next, the activated amino acids are added sequentially in the presence of coupling agents like HBTU, HATU, and DIC to form peptide bonds.
Repeat cycle: Deprotect and couple the successive amino groups to obtain the desired sequence.
Capping: The unreacted amine groups are then capped to prevent any deletion of the sequences.
Cleavage and Purification: Lastly, after the desired sequence is formed, the peptide sequence is cleaved from the resin with TFA, often having scavengers like water, TIPS, and EDT, to stabilize side-chain protecting groups. This crude product is further purified with HPLC and other methods.
This method is also compatible with automated peptide synthesizers and, therefore, well-suited for research and industrial applications.
Key Features of Fmoc-Glu(OtBu)-Thr[psi(Me,Me)Pro]-OH in Fmoc Amino Acid Chemistry
The Key features of Fmoc-Glu(OtBu)-Thr[psi(Me,Me)Pro]-OH are:
Increased Solubility: If the peptide sequence has more hydrophobic residues, it can form β-sheets. Also, the presence of hydrophobic residues decreases their solubility, making it difficult to purify. When Fmoc-Glu(OtBu)-Thr[psi(Me,Me)Pro]-OH is used, its solubility increases and helps you overcome problems with purification processes.
Scalability: This dipeptide molecule is well-suited for laboratory research and large-scale manufacturing units.
Repeatability: The Fmoc-Glu(OtBu)-Thr[psi(Me,Me)Pro]-OH ensures reproducible results by resulting in high yields and consistency across the batches.
Applications of Fmoc Peptides
The Fmoc peptides are the synthetic peptides synthesized using the Fmoc-based SPPS method. Owing to their versatility and ease of production, these peptides are used in the following areas:
Drug Discovery: A Peptide-based drug candidate is synthesized to study its interactions with biological targets. They are used in the developmental studies of therapeutic agents to treat cancer, diabetes, and autoimmune disorders.
Structural Biology: These synthetic peptides are used to study protein folding mechanisms, domain interactions, and post-translational modifications.
Therapeutics: These peptides help formulate stable peptide therapeutics, such as insulin analogs and GLP-1 receptor agonists.
Diagnostic studies: The Fmoc peptides are also used as biomarkers in diagnostic assays.
Biomedical Research: The Fmoc peptide is used to synthesize substrate analogs to study the enzyme-substrate reaction mechanism. It is also used to design synthetic antigens for the development of vaccines. The Fmoc peptides are used to produce peptide libraries for high-throughput screening of bioactive peptides. They are also employed to mimic protein’s active sites for studies.
Advantages of Using Fmoc-Glu(OtBu)-Thr[psi(Me,Me)Pro]-OH in Modern Peptide Synthesis
Highly efficient compound: Fmoc-Glu(OtBu)-Thr[psi(Me,Me)Pro]-OH reduces any chances of incomplete coupling and occurrence of any side reaction, thereby reducing waste.
Versatility: This amino acid derivative is applicable for all structural conformations, such as linear, cyclic and complex peptide structures.
High Total Yield: This dipeptide derivate produces peptide sequences with greater purity and fewer impurities.
Cost-effective: When Fmoc-Glu(OtBu)-Thr[psi(Me,Me)Pro]-OH is used for peptide synthesis, there is little to zero number of failed reactions, thus saving on the cost of synthesis.
Conclusion
As we conclude this discussion, it is essential to note that the strategic design of Fmoc-Glu(OtBu)-Thr[psi(Me,Me)Pro]-OH not only simplifies the synthesis of challenging sequences but also enhances the quality and efficiency of the final product.
This dipeptide is highly useful in meeting the increasing demands of the pharma and biopharma industry to scale up the production process quickly. At Advent, we provide high-quality Fmoc amino acids and other chemicals for your every study. Visit our website now for more information.
![Fmoc-Glu(OtBu)-Thr[psi(Me,Me)Pro]-OH: A Key Building Block in Peptide Synthesis](https://www.adventchembio.com/uploads/blogs/original_cover_images/fmoc-glu-thr.png)