Gas Chromatography (GC) is one of the most important techniques used in analytical chemistry to identify and quantify compounds. When combined with other techniques, this fundamental technique proves to be a powerful tool for industries like pharma, food, and environmental sciences.
The success of these highly sensitive techniques heavily depends on choosing a highly pure solvent. In this article, we underscore insights shared by Dr. Mohanty in his fifth Episode of Master Class. Dive into learn more!
What is Gas Chromatography? Understanding the Basics of GC and its solvents
Gas chromatography is used to separate the components in a mixture depending on the volatility and interaction with the stationary phase. This technique is highly sensitive and provides quick and precise results.
Here’s a Gas Chromatography Diagram to understand the process:
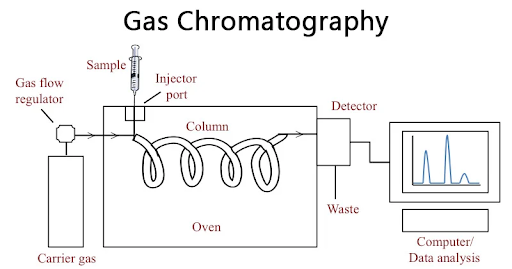
Steps involved in Gas Chromatography:
Sample Injection: A sample is injected via a calibrated microsyringe into the sample port. The sample may be either liquid, gas, or dissolved solid. There are different ways of introducing samples into the system, such as split injection, headspace sampling, and the latest method, autosamplers.
Vaporization: Next, the sample is vaporized at the injection port, and the sample vapours are then carried to the GC column by a carried gas, also known as the mobile phase.
Separation: As the analytes in the vapour form move through the GC column, the components get separated when interacting with the stationary phase. The components are separated depending on factors like boiling points, polarity, and molecular weight.
Signal Detection and Data Analysis: A compound is detected in the GC when it exits a column and generates a chromatogram with distinct peaks for every component. This chromatogram is then analyzed to identify the compounds in the mixture and quantify them.
Solvents used in GC
The choice of GC solvents depends on the type of sample, the GC method, the stationary phase and the detector.
Non-Polar Solvents: Hexane, Cyclohexane, and Toluene are examples of non-polar solvents used for separating non-polar or weakly polar samples to prevent their interaction with the stationary phase.
Polar Solvents: Methanol, Ethanol, and Acetonitrile are the commonly used polar solvents for separating polar samples. They are compatible with polar stationary phases.
Highly Volatile solvents: Solvents like acetone, ether, and pentane ensure quick evaporation in the injector port, leaving only the analyte for analysis.
Applications of GC
Owing to its sensitivity, this technique is applied across various fields to identify and quantify compounds in a mixture. Let’s study Gas Chromatography uses in detail:
Environmental Monitoring: GC is used to analyze the environmental pollutants in air, water, and soil. It can detect and quantify VOCs (Volatile Organic Compounds) and greenhouse gases like methane and other air pollutants.
GC is also applied to identify pesticides, herbicides, and other organic contaminants in potable water and wastewater.
This technique helps analyze hydrocarbons, pesticides, and other chemical residues contaminating the soil.
Food Quality Testing: Any pesticide or herbicide residues in fruits, vegetables, and grains can be detected using the Gas Chromatography technique. You can use GC to profile the flavour and fragrance of beverages, spices, and processed foods. The most critical application of GC is the detection of adulterants like melamine, formaldehyde, and synthetic dyes in food products, apart from identifying and quantifying preservatives like benzoates and sorbates.
Testing Drugs: The Drugs and their metabolites in biological fluids such as blood, plasma, and urine are quantified using GC. Because GC is a highly sensitive technique that can detect samples in trace amounts, it is used to detect substances like steroids, stimulants, and diuretics in athletes, as well as the identification of drugs, poisons, and alcohol levels in forensic investigations. In the pharmaceutical industry, Gas Chromatography ensures the purity of the drug products by detecting the presence of residual solvents, impurities, and side products.
Advanced Gas Chromatography Techniques: GC-MS, GC-HS, GC-TCD
In Ep 05, Dr. Mohanty explains that Gas Chromatography is a foundational analytical technique. When it is paired with advanced techniques, its versatility is expanded significantly. When GC is paired with MS, HS, and TCD, it brings unique capabilities in the following manner:
1. GC-MS (Gas Chromatography-Mass Spectrometry)
It combines the power of GC with the identification capabilities of Mass Spectrometry. GC separates the mixture, and the components are identified and quantified by Mass Spectrometer by calculating the mass-to-charge ratio (m/z).
Notable Advantages:
It detects the compounds and also provides structural details for precise identification.
GC-MS can detect trace amounts of analytes, which is ideal for environmental studies and forensic analysis.
MS produces a unique fragmentation pattern. These patterns can be analyzed to identify the unknown compounds.
2. GC-HS (Gas Chromatography-Headspace Analysis)
In this method, the volatile compounds are analyzed in the headspace without directly injecting the sample itself.
Notable Advantages:
It is a best-suited technique to analyze volatile and semi-volatile compounds without altering the sample matrix.
In this method, the risk of contamination or interference is minimal.
3. GC-TCD (Gas Chromatography with Thermal Conductivity Detector)
A Thermal Conductivity Detector can measure changes in the thermal conductivity due to the presence of analytes in the carrier gas. It is the most commonly used detector to detect compounds without chromophores.
Notable Advantages:
With GC-TCD, you can detect any compound, irrespective of its specific functional groups.
It provides precise quantification for bulk gases and permanent gases.
Since it does not destroy the sample, it can be used for further analysis.
Differences Between GC-MS, GC-HS, and GC-TCD
In this table, we have listed the key differences between these advanced analytical techniques.
How Gas Chromatography Solvents Impact Your Analytical Results?
A solvent used in GC acts as a carrier, a dissolution agent, and a medium for sample injection. A solvent ensures the analyte’s compatibility with the GC systems and maintains the integrity of the analysis. Here’s how a GC solvent impacts your results:
Purity: Using a highly pure solvent reduces the background noise and prevents ghost peaks. A highly pure GC solvent also prevents interference with the analyte’s detection.
Compatibility: Solvents work seamlessly with detectors like Flame Ionization Detectors (FID), Electron Capture Detector (ECD) or Mass Spectrometry (MS).
Non-reactive: Typically, a chemically inert solvent is used in GC to avoid reactions with the analytes or stationary phase, preserving the analyte's properties.
Volatility: A solvent to be used for GC must be highly volatile and easily eluted from the column before the analyte of interest to prevent overlapping peaks.
Advent ChemBio: Premium GC Solvent Solutions
At Advent, we offer over 50 gc solvents, including GC-MS solvents and GC-HS solvents for diverse analytical applications. Our High-purity solvents like methanol, dichloromethane, acetonitrile, and hexane are designed for maximum reliability and precision. The High-boiling solvents such as DMSO, DMF, NMP and DMA ensure thermal stability and consistent partitioning for headspace analysis.
Conclusion
Gas Chromatography proves to be a powerful tool in analytical chemistry. However, the success of this technique relies on selecting the right solvent. A solvent influences every aspect of GC, right from sample preparation to detection. Therefore, a solvent forms the backbone of a precise and reliable analysis.
Advent provides premium-grade GC solvents for a variety of analytical studies. To enhance your analytical precision, contact us at Advent now.