A detergent so good that it is used in the research labs as well as for pharma product formulations. Yes, you guessed it right, we are speaking of one of the most favourite and versatile detergents, Sodium lauryl sulphate.
In this blog, we shed light upon its chemical structure and properties, as well as its applications and precautions while handling. Lastly, we share the source to find your favourite chemical. Read further to learn more!
What is Sodium Lauryl Sulphate? Meaning and Definition
Sodium lauryl sulphate (SLS), also known as Sodium dodecyl sulphate (SDS), is an anionic surfactant mostly used in cleaning, cosmetic, and pharmaceutical products. Several industries leverage its excellent foaming, emulsifying, and detergent properties.
This chemical is found in white or off-white crystalline form or powdered form.
Understanding Sodium Lauryl Sulphate Structure
Chemical formula: C₁₂H₂₅NaO₄S or CH₃(CH₂)₁₁OSO₃Na
Molecular Weight: 288.38 g/mol
The following three structural components are responsible for sodium lauryl sulphate properties:
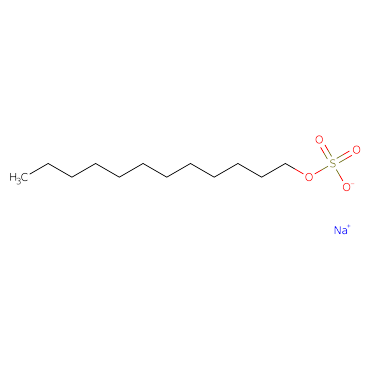
Hydrophobic Tail: The long alkyl chain of 12 carbons is responsible for its water-repelling property.
Hydrophilic Head: The polar sulphate functional group makes up its hydrophilic head.
Ion: The Sodium ion in its structure balances the negative charge of the sulphate group.
The hydrophilic head interacts with water and creates foam, while the hydrophobic tail is responsible for trapping oil and dirt. This structure makes it highly soluble in water, a strong foaming agent, and an effective emulsifier.
Key Sodium Lauryl Sulphate Uses in Lab and Industry
Owing to its structure and chemical properties, Sodium Lauryl Sulphate is a versatile detergent used in labs and industries for several purposes:
SDS-PAGE:
It is a technique to separate proteins based on their molecular weight. SDS is used to denature the protein and impart a uniform negative charge for the size-based migration through a polyacrylamide gel under the electric field.DNA/RNA Extraction:
Since DNA and RNA are the genetic materials present inside the nucleus of a cell. To extract this genetic material, the cell membrane must be lysed. SDS is used to prepare cell lysis buffer and extract the DNA/RNA. It is also used in the solubilization of proteins for biochemical analysis.Selective Media Preparation:
A selective media, like MacConkey-SDS Agar, allows the growth of selected species while inhibiting the growth of other unwanted bacteria. The presence of a thicker layer of Peptidoglycan in the gram-positive bacteria makes them susceptible to SDS agents. In contrast, the gram-negative bacteria are resistant to the antibacterial effect due to the presence of an outer membrane that protects them from disruption.
Glassware and Equipment Cleaning:
SDS is a strong surfactant and is, therefore, used to decontaminate lab equipment and glassware safely.Pharma Products:
As it improves the drug’s solubility, it is used as a wetting agent in tablet formulations. In the preparations of topical creams and ointments, it acts as an emulsifying agent.
Detergents:
It is one of the most essential ingredients in household and industrial cleaners, dishwashing liquids, laundry detergents, and surface cleaners.
How to Safely Handle Sodium Lauryl Sulphate: MSDS and Guidelines
Sodium lauryl sulfate is generally considered a safe chemical, but it may cause irritation and seriously impact the environment. Here are the guidelines to handle it safely:
Eyes and Skin: Exposure to skin for a long time may cause dryness, redness, and irritation. A person with eczema, dermatitis, or allergies must take more care while handling this chemical. If it comes in contact with the eyes, it can cause burning, stinging, and irritation. One must rinse one's eyes immediately with water. Also, in industrial settings, handling SDS is done using protective gloves and eyewear.
Accidental Inhalation: If you accidentally inhale the powdered SDS, it can cause respiratory irritation. Masks should be used when handling SDS in powdered form.
Environmental Impact: If Sodium lauryl sulfate is not discarded with appropriate precautions, it may end up in oceans and harm marine life.
Sodium Lauryl Sulphate Manufacturers in India: Key Players and Suppliers
Sodium lauryl sulphate, a key ingredient for various product formulations, must be purchased from a reliable supplier.
At Advent, we provide SLS of AR, LR, and HPLC grades and have more than 3000 products, including Fine & Speciality Chemicals, including inorganic salts, HPLC grade solids and solvents, Pharmacopoeia Grade Solvents, GC Grade Solvents, Ion-pairing reagents, electronic grade products, research chemicals and reagents, numerous building blocks, advanced intermediates, pharma impurities, and working standards.
We manufacture Pharma Grade Solvents under cGMP and comply with the regulatory standards set by IP, BP, USP, JP, Ph, and European specifications.
Sodium Lauryl Sulphate Price and Market Availability
This chemical is widely used and readily available in global markets, and its price depends on the purity and grade you want to purchase.
To purchase high-quality SLS, contact us today to get the details. Explore our website for more such fine and speciality chemicals.




